Neuroinflammation meets neurodegeneration
What drives neurodegeneration? How can it be halted? Neurodegeneration results with cognitive impairment and human suffering in millions of individuals worldwide. Targeting neurodegeneration therapeutically is challenging due to lack of understanding of the mechanisms driving the pathology, and due to lack of efficient ways to measure this in patients in vivo. The research group led by professor Laura Airas has investigated for the past 15 years the mechanisms contributing to neurodegeneration in a number of diseases including multiple sclerosis and parkinson’s disease, and developed here advanced multimodal methodology usable in treatment trials in conditions involving neuroinflammation and neurodegeneration.
Methodological approaches used by the Airas group include animal models, neuropathology, soluble biomarker analyses, metabolomics and advanced imaging including PET and MRI imaging such as Diffusion Tensor Imaging (DTI)-MRI and QSM (Quantitative Susceptibility Mapping). In image analysis, AI-based methods have been developed and applied. A major target for reducing the neurodegenerative processes in various neurological conditions is to decrease the widespread proinflammatory, neurotoxic glial activation in association with neurodegeneration.
Key words:
Neuroscience, PET-studies, multiple sclerosis, progression, Drug discovery, Clinical trials
Prior results of the research:
Among neurological conditions, development of MS treatment has been remarkably successful. The immune activity driving focal inflammation and relapses can be now practically halted. Yet, despite of this, many patients experience progression driven by ongoing neurodegeneration associated with smoldering, widespread inflammation driven by activated glial cells. While conventional MRI demonstrates sensitively the focal inflammatory lesions and is presently the cornerstone of MS diagnostics and clinical follow-up, it is poor at demonstrating the progression-related smoldering inflammation. PET imaging, on the other hand is a highly specific and versatile imaging method to assess more subtle pathological processes including smoldering inflammation in human brain in vivo at molecular level. A number of PET centers in Europe and USA drive the neuroscience field addressing the interplay between neuroinflammation and neurodegeneration, with Turku PET centre being one of the leading centers on the topic.
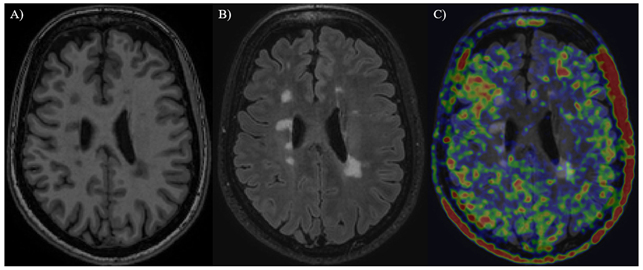
Brain of an MS patient. A) T1 MRI-image showing focal MS lesions as ”black holes” B) FLAIR MRI-image showing lesions as hyperintense bright areas C) PET-image of the brain with TSPO ligand [11C]PK11195
Main results of previous studies:
We have demonstrated that increased glial activation promotes disease progression and brain atrophy. We have developed methods to measure glial activation longitudinally in vivo. We have shown that some approved immunological therapies for MS reduce glial activation, but many do not, and new therapeutic approaches are thus needed to halt neuroinflammation-driven neurodegeneration. We are presently running treatment trials with novel compounds to reduce the harmful glial activation, and to slow down progression of neurodegenerative conditions, including progressive MS.
Collaboration:
We have wide national and international connections and collaborations with universities and pharma companies in Finland, Europe, United States and Australia. We are well funded by US and Finnish foundations, by US and Finnish government bodies, and by industry. We are affiliated with Neurocenter Finland and the Finnish Academy flagship InFLAMES (Innovation Ecosystem based on the Immune System). The PhD students in the group belong to Turku University Clinical Doctoral Program and the Drug Development Doctoral Program.
We look forward to hosting postdoctoral researcher(s) with an interest in neuroinflammation and neurodegeneration, and with a passion to do science in a fearless and professional way in a friendly environment with like-minded people in the happiest country in the world. Please contact professor Laura Airas by e-mail laura.airas@utu.fi to learn more.
Selected publications:
-
Sucksdorff M, Matilainen M, Tuisku J, Polvinen E, Vuorimaa A, Rokka J, Nylund M, Rissanen E, Airas L. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain. 2020 Dec 5;143(11):3318-3330. doi: 10.1093/brain/awaa275. PMID: 33006604; PMCID: PMC7719021.
-
Bezukladova S, Tuisku J, Matilainen M, Vuorimaa A, Nylund M, Smith S, Sucksdorff M, Mohammadian M, Saunavaara V, Laaksonen S, Rokka J, Rinne JO, Rissanen E, Airas L. Insights into disseminated MS brain pathology with multimodal diffusion tensor and PET imaging. Neurol Neuroimmunol Neuroinflamm. 2020 Mar 2;7(3):e691. doi: 10.1212/NXI.0000000000000691. PMID: 32123046; PMCID: PMC7136049.
-
Saraste M, Bezukladova S, Matilainen M, Tuisku J, Rissanen E, Sucksdorff M, Laaksonen S, Vuorimaa A, Kuhle J, Leppert D, Airas L. High serum neurofilament associates with diffuse white matter damage in MS. Neurol Neuroimmunol Neuroinflamm. 2020 Dec 8;8(1):e926. doi: 10.1212/NXI.0000000000000926. PMID: 33293460; PMCID: PMC7803327.
-
Bodini B, Tonietto M, Airas L, Stankoff B. Positron Emission Tomography in Multiple Sclerosis: Straight to the Target. Nat Rev Neurol. 2021. Nov;17(11):663-675. doi: 10.1038/s41582-021-00537-1.
-
Association of serum neurofilament light with microglial activation in multiple sclerosis Saraste M, Matilainen M, Vuorimaa A, Laaksonen S, Sucksdorff M, Leppert D, Kuhle J, Airas L. J Neurol Neurosurg Psychiatry. 2023 Sep;94(9):698-706. doi: 10.1136/jnnp-2023-331051.
- TSPO-Detectable Chronic Active Lesions Predict Disease Progression in Multiple Sclerosis Polvinen E, Matilainen M, Nylund M, Sucksdorff M, Airas LM. Neurol Neuroimmunol Neuroinflamm. 2023 Jun 22;10(5):e200133. doi: 10.1212/NXI.0000000000200133
Laura Airas
Professor
Turku PET center and Neurocenter
Investigators
Principal investigator:
Professor Laura Airas, MD, PhD
Senior investigators:
Maija Saraste, PhD
Markus Matilainen, PhD
Jussi Lehto MD, PhD
PhD students:
Marcus Sucksdorff, MD
Marjo Nylund, MSc
Amelie Möck, MD
Sini Laaksonen, MD
Imran Waggan, MSc
Olavi Misin, MD
Anna Vuorimaa, MD
Taru Nikkilä, MD
Eero Polvinen, MD
MSc students:
Matilda Kuusi
Venla Ahola
Extended medical studies:
Miika Manninen
Maija Jalava
Research coordinator:
Eveliina Honkonen, MSc
External funding
Academy of Finland
Turku University Hospital Clinical Funds (ERVA)
International Progressive MS Alliance
US National MS Society
IIS studies funded by industry
Collaborative projects with industry
Brain and whole-body insulin resistance in mild cognitive impairment and Alzheimer’s disease
Turku Alzheimer imaging group at the Turku PET Centre focuses on studying the effect of genetic and metabolic risk factors on memory disorders, especially Alzheimer’s disease. The metabolic study group at the Turku PET Centre, in turn, has conducted several studies exploring brain and whole-body glucose uptake during the gold standard measurement for the assessment of insulin resistance i.e. the hyperinsulinemic euglycemic clamp, for example in morbidly obese individuals and in individuals at risk for type 2 diabetes. Our most recent project combines the expertise of both groups at the Turku PET Centre and was set out to evaluate brain insulin resistance in mild cognitive impairment (MCI) and early Alzheimer´s disease.
We will utilize a whole-body PET/CT scanner (Vision Quadra, Siemens) to evaluate glucose metabolism simultaneously in the brain and the body both during a fasting state and during a hyperinsulinemic euglycemic clamp in early Alzheimer’s disease/MCI patients and cognitively healthy age-matched controls. The study protocol also includes brain amyloid-PET scans, MRI, comprehensive cognitive testing and analyzing blood biomarkers for Alzheimer’s disease.
18F-FDG-PET imaging during the hyperinsulinemic clamp enables evaluating insulin resistance in specific tissues of the body. Previous studies on Alzheimer’s disease animal models and on brain slices of individuals diagnosed with Alzheimer’s disease suggest that brain insulin resistance might play an important role in the neuropathological process of Alzheimer’s disease, but this phenomenon has not yet been studied in vivo in humans.
We are happy to host a postdoctoral researcher who has experience with PET imaging and modelling and an interest in memory disorders.
Key words:
Alzheimer’s disease, beta-amyloid, brain glucose uptake, Insulin resistance, Hyperinsulinemia, Euglycemic clamp, Type 2 diabetes, PET imaging, cognitive decline
Selected publications:
- Rebelos E, Latva-Rasku A, Koskensalo K, et al. Insulin-stimulated brain glucose uptake correlates with brain metabolites in severe obesity: A combined neuroimaging study. J Cereb Blood Flow Metab. 2023 Oct 12:271678X231207114. doi: 10.1177/0271678X231207114. Online ahead of print.
- Rebelos E, Bucci M, Karjalainen T, et al. Insulin Resistance Is Associated With Enhanced Brain Glucose Uptake During Euglycemic Hyperinsulinemia: A Large-Scale PET Cohort. Diabetes Care. 2021 Mar;44(3):788-794. doi: 10.2337/dc20-1549.
- Ekblad LL, Tuisku J, Koivumäki M, et al. Insulin resistance and body mass index are associated with TSPO PET in cognitively unimpaired elderly. J Cereb Blood Flow Metab. 2023 Sep;43(9):1588-1600. doi: 10.1177/0271678X231172519.
- Rebelos E, Rinne JO, Nuutila P, Ekblad LL. Brain Glucose Metabolism in Health, Obesity, and Cognitive Decline-Does Insulin Have Anything to Do with It? A Narrative Review. J Clin Med. 2021 Apr 6;10(7):1532. doi: 10.3390/jcm10071532.
- Rebelos E, Immonen H, Bucci M, et al. Brain glucose uptake is associated with endogenous glucose production in obese patients before and after bariatric surgery and predicts metabolic outcome at follow-up. Diabetes Obes Metab. 2019 Feb;21(2):218-226. doi: 10.1111/dom.13501.
- Ekblad LL, Johansson J, Helin S, et al. Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology. 2018 Mar 27;90(13):e1150-e1157. doi: 10.1212/WNL.0000000000005214.
Cutting Edge Clinical Neuroscience
Turku BrainLab focuses on investigating the neurobiological mechanisms of neurological and psychiatric disorders and translating this information towards new treatments. We work with several different clinical populations, causal brain lesions, state-of-the-art neuroimaging (MRI, PET, SPECT) and neuromodulation techniques (TMS, DBS, MRgFUS).
The neural origin of many neurological and psychiatric symptoms is still largely unclear. Neuromodulation techniques, such as transcranial magnetic stimulation (TMS), deep brain stimulation (DBS) and MR-guided focused ultrasound (MRgFUS), are increasingly used to treat brain disorders. However, use of neuromodulation is currently limited to only a minority of the brain disorders, because in most of the disorders we still don’t know where and how to modify brain function to treat the symptoms.
At Turku BrainLab, we try to solve this issue by leveraging unique clinical cohorts, causal brain lesions, state-of-the-art neuroimaging, and cutting-edge neuromodulation techniques. Our overarching aim is to develop new treatments for brain disorders, for which we are ideally positioned given our exceptional datasets, wide array of techniques and strong link to the university hospital. Our work covers a wide spectrum of brain disorders but has a special focus in movement disorders and addiction.
Neurological and psychiatric symptoms are caused by disruption of normal brain function, but in most cases the exact localization in the brain is not known. Identification of the neural origin of the symptoms is critical for the development of new therapeutic options. With the current technology, we can target almost any part of the brain using invasive or noninvasive neuromodulation techniques. For example, DBS and MRgFUS of the basal ganglia for movement disorders and repetitive TMS of the prefrontal cortex for depression have proven highly efficacious, far exceeding the efficacy of pharmacological treatments, and highlighting the therapeutic potential of these techniques. However, the main challenge and the reason why these techniques cannot be used to treat all brain disorders is that we do not know which part(s) of the brain the symptoms originate from and should be targeted with treatment.
Modern neuroimaging methods have allowed us to study the functions and malfunctions of the living human brain in greater detail than ever before. However, typical case-control studies are inherently limited by not being able to establish causal relationship between abnormal brain function and clinical symptoms. Thus, based on these studies, it is not possible to define which of the observed brain changes are causal and which are secondary/compensatory to the primary abnormality underlying the symptoms.
Throughout the history of neurology, studying brain lesions has formed the foundation for establishing causal relationships between brain damage and symptoms. However, in most cases, locations of lesions causing the symptom do not overlap, leaving the localization unclear. Accordingly, brain disorders are currently conceptualized as disorders of brain networks and new neuroimaging techniques, such as lesion network mapping and disconnectome mapping, have provided tools to localize brain lesion to networks (Joutsa et al. Curr Opin Neurol 2022; Joutsa et al. Brain 2023).
Together with our international colleagues, we have already mapped the causal brain circuits underlying, for example, parkinsonism (Joutsa et al, Brain 2018), dystonias (Corp et al., Brain 2019; Corp et al., Neurology 2022), tremor syndromes (Joutsa et al., Ann Neurol 2019; Younger et al., Neurology 2023), migraine (Burke et al. Brain 2020) and epilepsy (Schaper et al., JAMA Neurol 2023), and developed techniques to systematically identify treatment targets for neurological and psychiatric conditions (Joutsa et al., Ann Neurol 2018; Joutsa et al., Nat Med 2022). Combined with cutting-edge neuromodulation (TMS, DBS, MRgFUS) and neuroimaging (MRI, PET, SPECT) tools available at our hospital, we are ideally positioned to translate these findings towards new treatments.
Key words:
Neurology, Psychiatry, Neuroimaging, MRI, PET, SPECT, Lesion network mapping, Neuromodulation, TMS, DBS, MRgFUS
Examples of currently available datasets:
- Prospective lesion datasets: Our ongoing study is aimed to be the world largest prospective lesion network mapping study with currently more than 1000 patients with new-onset stroke enrolled. With a longitudinal follow up design, we collect data from more than 100 neurological and psychiatric symptoms, providing an exceptional dataset for lesion network mapping analyses.
- Retrospective lesion datasets: We have access to the electronic medical records and imaging data at Turku University Hospital, providing data from more than 20 years of patients treated at our hospital.
- Multicenter datasets: This ongoing effort, led by Dr. Joutsa, of the MDS post-stroke movement disorders study group is collecting the world largest dataset on post-stroke movement disorders together with 15 movement disorders centers around the world
- DBS-PET datasets: Using cervical dystonia as a proof-of-concept, this study aims to characterize the brain circuits responsible for symptom improvement. In the first of its kind study in Finland combining DBS and PET, we have mapped the neurobiological effects of the known highly efficacious neuromodulation treatment GPi-DBS (Honkanen et al. J Neurol Neurosurg Psychiatry 2023).
- TMS-PET datasets: These studies aim to create connectivity-based therapies using rTMS by combining rTMS with PET imaging. Our unpublished results show that connectivity-based rTMS can be used 1) to modulate the target network identified based on lesion network mapping, and 2) to modulate the dopamine system in a network-specific manner.
- Case-control datasets: Working at the boundaries of neurology and psychiatry, we have collected multiple imaging datasets (incl. epidemiological data, MRI, PET) on individuals with gambling disorder in both, general population and in individuals with Parkinson’s disease.
- Longitudinal cohorts: Our current datasets include a cohort of individuals with childhood-onset epilepsy followed up prospectively for more than 60 years and scanned repeatedly using MRI and PET, and retrospectively and prospectively identified patients with lesion-induced epilepsy. Combined, these datasets provide unique insights into the neurobiological mechanisms and very long-term outcomes of epilepsy.
Turku BrainLab:
In addition to the available and upcoming exceptional datasets, the unique strength of Turku BrainLab is interdisciplinary and multimodal research, combining several fields with a strong link between clinical and research work. Our 30+ person international team includes researchers with backgrounds in clinical neurology, psychiatry and radiology, human neuroscience, engineering, and nuclear medicine. The director of Turku Brainlab is Dr. Juho Joutsa who is a tenured full professor of neurology at University of Turku, chief neurologist at Turku University Hospital, and the chair of Turku Brain and Mind Center. Dr. Joutsa completed his postdoctoral training at Massachusetts General Hospital and Harvard Medical School. He is the youngest professor of neurology in Finland, has received several awards (incl. MD thesis supervisor of the year and young investigator awards from both neurology and medicine), and has been listed among the 100 most influential people in medicine in Finland in 2022.
We can host postdocs with strong background in human neuroscience, neuroimaging or data analytics, to join our dynamic and young lab. We are guided by the pursuit of academic excellence and make every effort possible to provide you with a major steppingstone for your own career in academia and beyond. Dr. Joutsa will act as your primary supervisor and mentor. We would be happy to discuss with you your research idea(s) and proposal in more detail. Please, don’t hesitate to reach out if you have any questions or are interested in joining our lab.
Selected publications:
- Niemi KJ, Huovinen A, Jaakkola E, Glerean E, Nummenmaa L, Joutsa J. Bodily maps of symptoms and emotions in Parkinson’s disease. Mov Disord. 2024;39(6):1037-1043.
- Honkanen EA, Rönkä J, Pekkonen E, Aaltonen J, Koivu M, Eskola O, Eldebakey H, Volkmann J, Kaasinen V, Reich MM, Joutsa J. GPi-DBS induced brain metabolic activation in cervical dystonia. J Neurol Neurosurg Psychiatry 2024:95(4):300-308.
- Joutsa J, Lipsman N, Horn A, Cosgrove GR, Fox MD. The return of the lesion for localization and therapy. Brain 2023;146(8):3146-55.
- Schaper F, Nordberg J, Cohen AL, Lin C, Hsu J, Horn A, Ferguson MA, Siddiqi SH, Soussand L, Winkler A, Simo M, Bruna J, Rheims S, Guenot M, Bucci M, Nummenmaa L, Staals J, Colon A, Ackermans L, Bubrick E, Peters J, Wu O, Rost N, Grafman J, Blumenfeld H, Temel Y, Rouhl R, Joutsa J, Fox MD. Lesion-related epilepsy maps to a common brain network. JAMA Neurol 2023;80(9):891-902.
- Corp DT, Greenwood C, Morrison-Ham J, Pullinen J, McDowall G, Younger E, Jinnah H, Fox MD, Joutsa J. Clinical and structural findings in patients with lesion-induced dystonia: descriptive and quantitative analysis of all published cases. Neurology 2022;99(18):1957-67.
- Joutsa J, Moussawi K, Siddiqi S et al. Brain lesion disrupting addiction map to a common human brain circuit. Nature Medicine 2022;28:1249-55.
- Jaakkola EA, Huovinen A, Kaasinen V, Joutsa J. No change in prevalence of impulse control disorders during the last decade. Mov Disord. 2021;36(2):521-3.
- Joutsa J, Shih LC, Fox MD. Mapping Holmes’ tremor circuit using the human brain connectome. Ann Neurol 2019;86(6):812-20.
- Corp DT, Joutsa J, Darby RR, Delnooz CCS, van den Warrenburg BPC, Ren J, Batla A, Bhatia KP, Jinnah HA, Liu H, Fox MD. Network localization of cervical dystonia from causal brain lesions. Brain 2019;142(6):1660-74. Joutsa J, Horn A, Hsu J, Fox MD. Localization of parkinsonism based on focal brain lesions. Brain 2018;141(8):2445-56.
- Joutsa J, Shih LC, Horn A, Reich MM, Wu O, Rost N, Fox MD. Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol. 2018;84(1):153-7.
- Joutsa J, Rinne JO, Hermann B, Karrasch M, Anttinen A, Shinnar S, Sillanpää M. Association between childhood-onset epilepsy and amyloid burden 5 decades later. JAMA Neurology 2017;74(5):583-90.
Advancing Clinical Research in Movement Disorders and Neurology
We offer a dynamic research environment, with access to advanced neuroimaging and clinical tools, and collaboration opportunities within a leading European neuroscience network.
The research group led by Prof. Valtteri Kaasinen is offering a unique opportunity for a Postdoctoral Researcher with a strong clinical background in neurology and movement disorders. Our research focuses on understanding and treating neurological diseases such as Parkinson’s disease, essential tremor, progressive supranuclear palsy (PSP), multiple system atrophy (MSA), and other neurodegenerative conditions. We are particularly interested in candidates who are passionate about clinical phenomenology and treatment approaches, with potential for involvement in neuroepidemiological research. Experience in neuroimaging and/or neuropathology is a plus.
Key responsibilities:
- Lead clinical research projects focusing on the phenomenology, diagnosis, and treatment of movement disorders.
- Collaborate with a multidisciplinary team, including neurologists, neuroscientists, and other clinical specialists.
- Analyze clinical, neuroimaging, and epidemiological data to derive meaningful insights into movement disorders.
- Publish high-impact research articles and present findings at international conferences.
- Optionally engage in neuroepidemiology and apply population-based approaches to movement disorder research.
Required Qualifications:
- PhD or MD in neurology, movement disorders, or a related clinical field.
- Strong interest and/or experience in clinical research and treatment of movement disorders.
- Neuroimaging experience (e.g., MRI, PET) is a plus, especially if combined with movement disorder research.
- Knowledge of neuropathology is also an advantage.
- Experience in neuroepidemiology is desirable but not required.
- Proven track record of scientific publications and clinical research.
Selected publications:
- Saarinen EK, et al. (2024). Dietary Caffeine and Brain Dopaminergic Function in Parkinson’s Disease. Ann Neurol 96:262-275
- Saari L, et al. (2021). Depression and Nigral Neuron Density in Lewy Body Spectrum Diseases. Ann Neurol 89:1046-1050
- Kuusimäki T, et al. (2021). Increased Risk of Parkinson’s Disease in Patients with Schizophrenia Spectrum Disorders. Mov Disord 36:1353-1361
- Honkanen E, et al. (2019). No Link Between Striatal Dopaminergic Axons and Dopamine Transporter Imaging in Parkinson’s Disease. Mov Disord 34:1562-1566
- Saari L, et al. (2017). Dopamine Transporter Imaging Does Not Predict the Number of Nigral Neurons in Parkinson’s Disease. Neurology 88:1461-1467
Valtteri Kaasinen
Professor of Neurology, Chief Physician
Department of Clinical Neurosciences, Faculty of Medicine, University of Turku
Neurocenter, Turku University Hospital
Developmental neuroscience of early life exposures, genes and environment
FinnBrain Neuroimaging Lab
We study how genes and environment, especially early life exposures, shape the brain structure and function. We are interested in how these, often subtle individual differences explain later characteristics, skills and health of the children. We are part of FinnBrain birth cohort study (https://sites.utu.fi/finnbrain/en/) that was launched at the University of Turku in 2010, and its purpose is to study the combined influence of environmental and genetic factors on child development and later health outcomes. The follow-up of the children will continue for several decades. The research is multidisciplinary and has extensive national and international collaboration. The participants are families from the city of Turku, municipalities in Turku area, and Åland Islands.
Key words:
Neuroimaging, MRI, EEG, fNIRS, early life exposures, maternal health, brain development.
Our main research activities:
- 1. FinnBrain Birth Cohort study was launched at the University of Turku in 2010, and its purpose is to study the combined influence of environmental and genetic factors on child development and later health outcomes. It is an ongoing study and we take care of future data collections and are enthusiastically analysing the data that we have accumulated from prenatal period over the first five years of life.
- 2. The Centre of Excellence for Learning Dynamics and Intervention Research (InterLearn) investigates the links between children’s learning and mental health, and the factors that explain the effectiveness of learning support interventions. We are part of InterLearn and we will be working with synergistic and partly harmonised data sets together with investigators from the University of Jyväskylä.
- 3. Population neuroscience of the neural correlates of obesity and future obesity risk in (pre)adolescents is one the more recent research initiatives for us: it “endeavours to identify environmental and genetic factors that shape the function and structure of the human brain; it uses tools and knowledge of genetics, epidemiology, and cognitive neuroscience.” We use data from the ABCD Study to enable these studies and have also extended many of the brain network modelling approaches to infant MRI data stemming from the developing Human Connectome Project.
- 4. Lifespan neuroscience is a new initiative that has just started. We are part of the Lifespan consortium in creation of brain growth charts for additional neuroimaging measures to accompany the volumetric growth charts that were recently created.
We seek new investigators with interest in multidisciplinary research and multimodal neuroimaging. We especially value skills in MRI, EEG and fNIRS and experience carrying out studies with small intense follow up data and / or big data analyses. Related strong skills in statical analyses and / or machine learning, and basic programming skills are also highly valued.
PIPARI Project: Follow-up study of preterm infants
The PIPARI Study has followed a group of very preterm infants and their healthy fullterm controls up to 17 years of age. The multidisciplinary follow up was completed in spring 2024 resulting in a rich data base extending from fetal life through neonatal period and childhood into young adulthood. Extensive assessments have been performed at term equivalent age, at 2 years of corrected and at 5 years, 11 years and at 17 years of age including brain MRI at term equivalent age and fMRI at 13 years of age. The study group includes over 200 very preterm infants with their sex-matched healthy full-term controls.
Our vision is to identify protective factors for good functional outcome. Sophisticated statistical analyses will be applied to find trajectories, risk factors and protective factors for multidimensional functional outcomes. Outcomes are classified under the themes of academic performance, psychiatric symptoms, physical health and maturation, and resilience.
The research is carried out at Turku University Hospital. We are a group of researchers and clinicians from different disciplines including pediatrics and neonatology, child neurology, developmental psychology and child/adolescence psychiatry, speech pathology, and radiology. More information please find www.utu.fi/pipari.
We seek for candidates with understanding of child development and advanced skills in data analysis to be applied to data from different age points and several domains. We offer a unique data set for analyses. PhD degree in e.g. medicine, biomedicine and psychology are of high value.
Key words:
Preterm infant, development, cognition, behavior, quality of life, MRI, ultrasound
Liisa Lehtonen
Professor
Investigators
Principal investigators:
Professor Liisa Lehtonen, MD, PhD
Professor Leena Haataja, MD, PhD
Docent Helena Lapinleimu, MD, PhD
Senior investigators and postdocs:
Professor Päivi Rautava, MD, PhD
Professor Riitta Parkkola, MD, PhD
Professor Riikka Korja, PhD
Professor Max Karukivi, MD, PhD
Associate Professor Suvi Stolt, PhD
Docent Sirkku Setänen, MD, PhD
Mira Huhtala, MD, PhD
Milla Ylijoki, MD, PhD
Anna Nyman, PhD
Petriina Munck, PhD
Virva Saunavaara, PhD
PhD students:
Tuomo Lehtonen
Susanna Salomäki
Tiina Saarinen
Linda Grönroos
Eveliina Joensuu
Minttu Helin
Laura Haveri
Eeva Mäkilä
Research coordinator and other research personnel:
Helena Ollila, statistician
Sofia Sapattinen, coordinator
Human Emotion Systems Laboratory
Human Emotion Systems Laboratory at Turku PET Centre is a leading European group in systems-level research in emotions and their disorders. Our work focuses on novel concept of imaging the brain-periphery axis using total-body positron emission tomography and state-of-the art functional and structural magnetic resonance imaging.
We have a strong background in human emotion science, computational approaches for resolving brain basis of higher mental functions as well as state-of-the art methodology in systems-level imaging of total-body biological circuits in health and disease. We focus also on development of state-of-the art methodological solutions for large-scale medical image analyses and multi-level integrative analysis of metabolic functions in the brain and periphery.
The studies are done in the context of affect regulation in health and disease as well as cardiometabolic health and systems neuroscience.
In our group, we value skills in molecular and structural imaging (PET / MRI) as well as application of advanced statistical techniques and signal analysis methods for PET and MRI data. Also, we appreciate a background and PhD degree in medicine, psychology, computational science, physics, or related fields, and skills for working with complex empirical data. Programming skills (MATLAB, Python, or comparable languages) are essential.
Key words:
Brain-periphery axis, Human Emotion Systems, Systems neurosicience, Cardiometabolism
Psychiatric symptoms and disordered sleep as risk factors for memory disorders
The research of the Turku Mood and Memory Lab focuses on the connections between psychiatric disorders and symptoms, such as depression and anxiety, and neurodegenerative diseases, especially Alzheimer’s Disease. By utilizing both the invaluable information gathered by the FinnBrain Birth Cohort study as well as diverse brain imaging datasets and the use of cutting-edge analysis methods, we aim to produce valuable scientific information that will aid in early risk detection and prevention of memory disorders. One key area of interest are the structural brain alterations seen in depression, mild cognitive impairment and Alzheimer’s disease. Further, we strive to move on to clinical intervention studies to rehabilitate cognitive symptoms of major depressive disorder, as we do not only believe that depression may predispose a person to later memory disorders through cognitive scarring, but also to ameliorate the prognosis of depression itself. Altogether, we aim to produce invaluable new knowledge on disorders with an especially high disease burden; mood and memory disorders.
The phenomena are explored in three datasets: young and predominantly healthy adults in the FinnBrain cohort, elderly MCI subjects taken from a PET imaging dataset, and large MR imaging datasets of patients with memory impairments taken from a biobank study. The project will explore how these conditions relate to each other through neuroimaging analyses, psychological assessments, patient records, family health histories, and different biological markers from subject subgroups.
In FinnBrain (data set I), we investigate the association between a family history of memory disorder and mental health symptoms in 2178 adults. A comprehensive family history of memory disorders has previously been collected from the subjects, and a longitudinal survey of various psychological symptoms (including depressive and anxiety symptoms) has been conducted over a nine-year period. In addition, biological samples (DNA, cytokine, and cortisol samples) collected from a subsample of the subjects will be used to explore the mechanisms of this association. The PET data (data set II) will be re-analyzed from previously imaged elderly memory-impaired (MCI) patients’ 11C-PIB and 18F-FDG PET brain images (n= ca. 100) to determine whether tracer accumulations differ depending on depressive or other psychiatric and/or sleep disorder history. Thirdly (data set III), we are participating in the establishment of a brain image biobank (IMAGEN project) and include a pilot data set of memory disorder patients (n= ca. 3000) to address the research question: are the parameters measured from MR images (e.g. regional atrophy, so-called brain age) different depending on the possible history of depression.
The principal investigator has extensive experience in both memory disorders and psychiatric syndromes and solid expertise in imaging and other biomarker research methodologies. Additionally, the team has excellent networks to strengthen this new and unique line of research.
Our lab is looking to welcome postdoctoral researchers who have a background in psychiatric epidemiology, neuropsychology, human neuroscience, neuroimaging, or data analytics. An interest in the associations between psychiatric symptoms and memory decline and/or neurodegeneration is warranted. As a young and dynamic team, we offer great opportunities for your career advancement. Associate professor Scheinin will serve as your main supervisor and mentor throughout your stay. If you have any questions or are interested in becoming a part of the Turku Mood and Memory Lab, please feel free to contact us!
Key words:
Depression, Mood Disorders, Psychiatric Symptoms, Neuroscience, Alzheimer’s Disease, Mild Cognitive Impairment, Register Studies, Psychiatric Epidemiology, Neuroepidemiology, Psychiatry, Neurodegeneration, MRI, PET, Cognitive Decline
Selected publications:
The Turku Mood and Memory Lab is currently working on the first papers to be published under its name. Here are some of the (70+) publications of the principal investigator Scheinin during the last 10 years:
- Korja R, Nolvi S, Scheinin NM, Tervahartiala K, Carter A, Karlsson H, Kataja EL, Karlsson L. Trajectories of maternal depressive and anxiety symptoms and child's socio-emotional outcome during early childhood. J Affect Disord. 2024 Mar 15;349:625-634.
- Merisaari H, Karlsson L, Scheinin NM, Shulist SJ, Lewis JD, Karlsson H, Tuulari JJ: Effect of number of diffusion encoding directions in neonatal diffusion tensor imaging using Tract-Based Spatial Statistical analysis. Eur J Neurosci. 2023 Aug 29.
- Lehtola SJ, Tuulari JJ, Karlsson L, Lewis JD, Fonov VS, Collins DL, Parkkola R, Saunavaara J, Hashempour N, Pelto J, Lähdesmäki T, Scheinin NM, Karlsson H: (2022) Sex-specific associations between maternal pregnancy-specific anxiety and newborn amygdalar volumes - preliminary findings from the FinnBrain Birth Cohort Study, Stress 2022; 25:213-226
- Lund RJ, Kyläniemi M, Pettersson N, Kaukonen R, Konki M, Scheinin NM, Karlsson L, Karlsson H, Ekholm E: Placental DNA methylation marks are associated with maternal depressive symptoms during early pregnancy. Neurobiol Stress. 2021;15:100374.
- Lehtola S, Tuulari JJ, Scheinin NM, Karlsson L. Parkkola R, Merisaari, Lewis JD, Fonov VS, Collins DL, Evans AC, Saunavaara J, Hashempour N, Lähdesmäki T, Acosta H, Karlsson H: Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. Neuroimage Clin. 2020;28:102380.
- Scheinin NM, Gardberg M, Röyttä M, Rinne JO: Negative 11C-PIB PET predicts lack of Alzheimer's Disease pathology in postmortem examination. J Alzheimers Dis. 2018;63:79-85.
- Karlsson L, Tolvanen M, Scheinin NM, Uusitupa H-M, Korja R, Ekholm E, Tuulari JJ, Pajulo M, Huotilainen M, Paunio T, Karlsson H, and the FinnBrain Birth Cohort Study Group: Cohort profile: The FinnBrain Birth Cohort Study (FinnBrain). Int J Epidemiol. 2018;47:15-16j
- Kajanoja J, Scheinin NM, Karlsson L, Karlsson H, Karukivi M: Illuminating the clinical significance of alexithymia subtypes: A cluster analysis of alexithymic traits and psychiatric symptoms. J Psychosom Res. 2017;97:111-117.
- Scheinin NM, Wikman K, Jula A, Perola M, Vahlberg T, Rokka J, Någren K, Viitanen M, Rinne JO. Cortical ¹¹C-PIB uptake is associated with age, APOE genotype, and gender in "healthy aging". J Alzheimers Dis. 2014;41:193-202.
- Kemppainen NM, Scheinin NM, Koivunen J, Johansson J, Toivonen JT, Någren K, Rokka J, Karrasch M, Parkkola R, Rinne JO. Five-year follow-up of 11C-PIB uptake in Alzheimer's disease and MCI. Eur J Nucl Med Mol Imaging. 2014;41:283-289.
