Development of novel PET radiotracers for immunoPET imaging of heart and brain
The recent advances in antibody engineering has made it possible to construct antibodies with ultimate target specificity and tailored pharmacokinetics. Diagnostic immune PET imaging of cancer was one of the first utilizers of these novel technologies, but recently, besides oncology, novel applications have emerged such as immune PET imaging of CNS diseases. Our research groups utilize the most recent technologies in antibody engineering and PET radiochemistry to develop pharmacokinetically tailored recombinant antibody fragments for PET imaging of target proteins in the heart and brain. Our special interests relate to studying the role of GABA-A receptor subtypes in the health of heart and brain and exploring the possibilities of novel pretargeted PET radiopharmaceuticals.
Key words:
ImmunoPET, PET imaging, PET radiotracers, Pharmacokinetics, Heart health, Brain health
Prior results of the research:
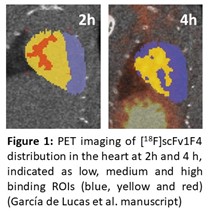
By joined research efforts of Dr. Lopez Picon, Prof. Airaksinen and Prof. Lamminmäki, we have successfully created the 1st generation of novel antibody fragment based tracers against GABA-A receptors. GABA-A receptors are ion channels, which are expressed in the brain and in several peripheral organs, including the heart. Structurally, the GABA-A receptors are formed by five protein subunits, and they are classified according to their subunit composition. The GABA-A receptor subtypes exhibit region specific distributions in the brain and other organs. In the CNS, the receptor subtypes have shown different functional and physiological roles and to mediate variety of pharmacological effects, but in other organs, such as in the heart, their differential roles are less explored. [18F]scFv1F4 and [89Zr]di-scFv1F4 were developed for imaging GABA-A a1 receptor subtype in peripheral organs and in the brain. From these the bispecific [89Zr]di-scFv1F4 was able to cross the blood-brain barrier. The single fragment, [18F]scFv1F4, exhibited favorable pharmacokinetics and heterogenous distribution in the myocardium, with highest activity 2h after the injection (Figure 1).
The candidate should have:
- PhD in a suitable field (chemistry, radiochemistry, medicinal chemistry, biochemistry or biotechnology)
- Previous research experience in new radiotracer development. In addition, expertise in recombinant protein expression and/or purification is deemed advantageous.
Selected publications:
- 1. Ángel García de Lucas, Urpo Lamminmäki, and Francisco R. López-Picón. 2023. "ImmunoPET Directed to the Brain: A New Tool for Preclinical and Clinical Neuroscience" Biomolecules, 2023, 13, no. 1: 164.
- 2. Tatsiana Auchynnikavaa, Antti Äärelä, Heidi Liljenbäck, Juulia Järvinen, Putri Andriana, Luciana Kovacs, Jarkko Rautio, Johan Rajander, Pasi Virta, Anne Roivainen, Xiang-Guo Li, Anu J. Airaksinen. Tetrazine glycoconjugate for pretargeted PET imaging of trans-cyclooctene functionalized molecular spherical nucleic acids. ACS Omega, 2023, https://doi.org/10.1021/acsomega.3c04041
- 3. Dave Lumen, Danielle Vugts, Marion Chomet, Surachet Imlimthan, Mirkka Sarparanta, Ricardo Vos, Maxime Schreurs, Mariska Verlaan, Pauline Lang, Wissam Beaino, Albert D. Windhorst, Anu J. Airaksinen. Pretargeted PET imaging with a TCO-conjugated anti-CD44v6 chimeric mAb U36 and [89Zr]Zr-DFO-PEG5-Tz. Bioconjugate Chemistry, 2022, 33, 956-968
Anu Airaksinen
Professor
Turku PET Centre/Department of Chemistry
Urpo Lamminmäki
Professor
Department of Life Technologies
Ultrasensitive immunoassays for neurological biomarkers
Currently the practical use of protein biomarkers for neurological diseases is significantly limited due to their presence in blood at hardly detectable or totally undetectable levels using conventional immunoassay technologies, which are routinely used for other biomarkers. The neurological biomarkers can be studied using cerebral spinal fluid, but measurement of proteins leaking through the blood-brain barrier would enable significantly less invasive blood sampling. Thus, the availability of ultrasensitive immunoassays would facilitate convenient testing and support development of neurological biomarkers to earlier diagnostics of neurological disorders.
We have developed in collaboration with European wide research network a new superior luminescent reporter technology, photon upconversion luminescence, and demonstrated its capability to ultrasensitive detection at conventional immunoassay platform matching the sensitivity of even the most complex digital immunoassays based on special instrumentation. Photon upconversion is based on trivalent lanthanide doped nanomaterials capable of stacking the energy of two absorbed low-energy near-infrared photons to emit a single high-energy photon at visible wavelengths. The resulting anti-Stokes photoluminescence, which is measurable using inexpensive epifluorescence setup, enables total elimination of background autofluorescence and results in exceptionally low limit of detection in immunoassays for protein biomarkers.
The limit-of-detection in photon upconversion luminescence-based assays is still fundamentally restricted by the specific binding strength of the employed antibodies and the non-specific binding of the reporter-antibody conjugates. To enable further improvement of the assay sensitivity we are currently studying active techniques and countermeasures to reduce and eliminate the signal originating from the non-specific interactions of the reporter-antibody conjugates. These techniques could enable a breakthrough in the development of ultrasensitive immunoassays using photon upconversion luminescence.
Key words:
Digital immunoassay, In vitro diagnostics, Diagnostic discovery, Neurological disorders, Neurological biomarkers, Blood test limit-of-detection, Blood-brain barrier leakage, photon upconversion luminescence
Selected publications:
- What digital immunoassays can learn from ambient analyte theory: a perspective. Hans H Gorris, Tero Soukka. Anal Chem 2022 94:6073-6083. doi: 10.1021/acs.analchem.1c05591.
- Supersensitive photon upconversion based immunoassay for detection of cardiac troponin I in human plasma. Raiko, K., Lyytikäinen A, Ekman M, Nokelainen A, Lahtinen S, Soukka T. Clin Chim Acta. 2021; 523:380-385. doi: 10.1016/j.cca.2021.10.023.
- Effect of particle size and surface chemistry of photon-upconversion nanoparticles on analog and digital Immunoassays for cardiac troponin. Brandmeier JC, Raiko K, Farka Z, Peltomaa R, Mickert MJ, Hlaváček A, Skládal P, Soukka T, Gorris HH. Adv Healthc Mater. 2021; 10:e2100506. doi: 10.1002/adhm.202100506.
- Improving the sensitivity of immunoassays by reducing non-specific binding of poly(acrylic acid) coated upconverting nanoparticles by adding free poly(acrylic acid). Lahtinen S, Lyytikäinen A, Sirkka N, Päkkilä H, Soukka T. Mikrochim Acta. 2018; 185:220. doi: 10.1007/s00604-018-2756-z.
In vitro diagnostic tests for cardiac biomarkers
Laboratory tests are being used around the world for diagnosing cardiac diseases and estimating the risk for adverse cardiac events. However, these tests are not optimal and tests with better clinical specificity and sensitivity are needed to improve patient care. At the Cardiac Biomarker Laboratory, we aim to develop laboratory tests for novel cardiac biomarkers that will have significant impact on how cardiac diseases are diagnosed and treated.
The Cardiac Biomarker Laboratory of the Biotechnology Unit at the Department of Life Technologies is focused on developing highly sensitive immunoassays for novel cardiac biomarkers and investigating the clinical use of these biomarkers for diagnosis and risk prediction of cardiac diseases. The group, led by Assistant Professor Saara Wittfooth, applies the innovative label technologies developed at the Biotechnology Unit to develop unique assays that are not available in any other laboratory (examples of such assays are the assays for long cardiac troponin T, free PAPP-A and cardiac troponin autoantibodies). The work includes developing immunoassays in various platforms by designing antibody combinations and optimizing assay conditions and procedures. The work involves close collaboration with cardiologists around the world.
Lately the research of the Laboratory has been focused on different forms of cardiac troponins. Tests for cardiac troponins (cardiac troponin I and cardiac troponin T, cTnT) are widely used for diagnosing myocardial infarction around the world. However, the high sensitivity troponin tests that are currently used in the hospitals produce elevated results also in many other situations than myocardial infarction (such as impaired kidney function, atrial fibrillation, severe infections, strenuous physical exercise). Therefore, there is a high demand for a more specific diagnostic test for myocardial infarction. We have developed in the Cardiac Biomarker Laboratory a novel simple immunoassay-based test that detects only the long forms of cTnT, while the tests being used at hospitals detect the long and short forms of cTnT. Our first studies have shown very promising results, as the long cTnT test was able to differentiate between myocardial infarction patients and end-stage renal disease patients much better than the troponin test currently in use at hospitals.
Another research topic gaining more interest after a while in the Cardiac Biomarker Laboratory is the cardiac troponin autoantibodies (cTnAAb). cTnAAb, which have been found in the blood of myocardial infarction patients but also in healthy individuals, can cause negative interference in immunoassays by masking the epitopes of the assay antibodies. An assay to detect cTnAAb in patient samples was previously developed in the Laboratory. Lately there has been increasing interest in the scientific community towards macrotroponins, circulating complexes of troponins and cTnAAb, which may cause positive interference due to reduced clearance. We are now interested in examining macrotroponins with the available cTnAAb assay as well as with other methods in various patient groups.
Key words:
In vitro diagnostics, Myocardial infarction, Cardiac biomarkers, Cardiac troponins, Troponin autoantibodies
The candidate should have:
- PhD in a suitable field (biotechnology, biochemistry, laboratory medicine etc.)
- Extensive experience in performing and developing immunoassays
- Ability to communicate fluently in English
- Excellent scientific writing skills
Selected publications:
- Airaksinen KEJ, Aalto R, Hellman T, Vasankari T, Lahtinen A, Wittfooth S. Novel Troponin Fragmentation Assay to Discriminate Between Troponin Elevations in Acute Myocardial Infarction and End-Stage Renal Disease. Circulation. 2022 Nov;146(18):1408-1410. doi: 10.1161/CIRCULATIONAHA.122.060845
- Savukoski T, Ilva T, Lund J, Porela P, Ristiniemi N, Wittfooth S, Pettersson K. Autoantibody prevalence with an improved immunoassay for detecting cardiac troponin-specific autoantibodies. Clin Chem Lab Med. 2014 Feb;52(2):273-9. doi: 10.1515/cclm-2013-0310.
- Savukoski T, Twarda A, Hellberg S, Ristiniemi N, Wittfooth S, Sinisalo J, Pettersson K. Epitope specificity and IgG subclass distribution of autoantibodies to cardiac troponin. Clin Chem. 2013 Mar;59(3):512-8. doi: 10.1373/clinchem.2012.194860.
